Gilead’s new two HIV vaccinations get FDA approval
The FDA allows AI tools to predict breast cancer risk
Senior Medical Analyst Dr. Mark Siegel discusses the future risks of breast cancer and advances in artificial intelligence aimed at predicting an increase in health risks from cannabis with users age.
newYou can listen to Fox’s news articles!
The US Food and Drug Administration (FDA) has approved two new shots annually (the first and only shot of its kind). Prevents HIVdrug creator Gilead Science announced on Wednesday.
The company’s injectable HIV-1 CapSID inhibitor (Lenacapavir), sold under the name Yeztugo, is Sexually acquired HIV For adults and young people.
“This is a historic day of decades of fighting against HIV,” said Daniel O’day, CEO of California-based Gilead Sciences, in a press release.
Alzheimer’s disease can be prevented by antiviral drugs already on the market
The medications that need to be managed only twice a year “shows amazing results. Clinical research“As Gilead claims, it can change HIV prevention.
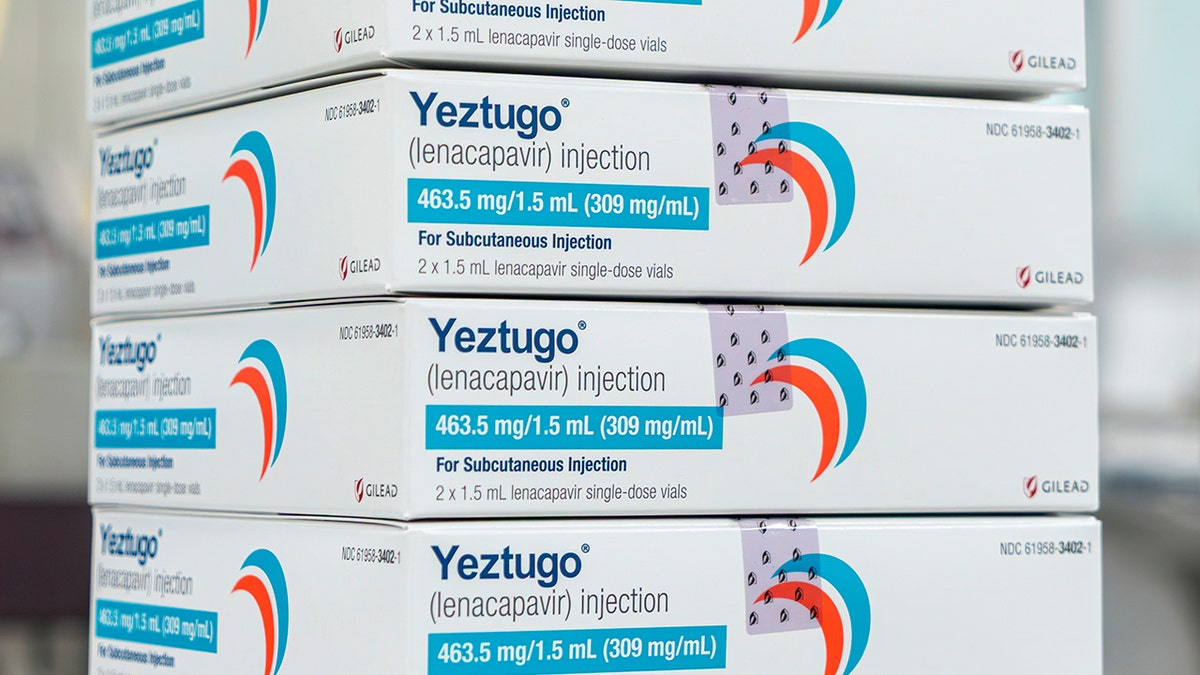
The U.S. Food and Drug Administration approved Yeztugo, two shots a year, to prevent HIV, the creator of the drug, announced Wednesday. (Gilead Sciences via AP)
This drug is administered as injectable under the skin that the body slowly absorbs. Individuals should undergo a negative HIV-1 test before starting treatment.
Click here to get the Fox News app
A large trial last year proved that the drug is not only nearly 100% effective in preventing HIV, but also superior to a day-to-day oral drug like Truvada, another drug by Gilead.
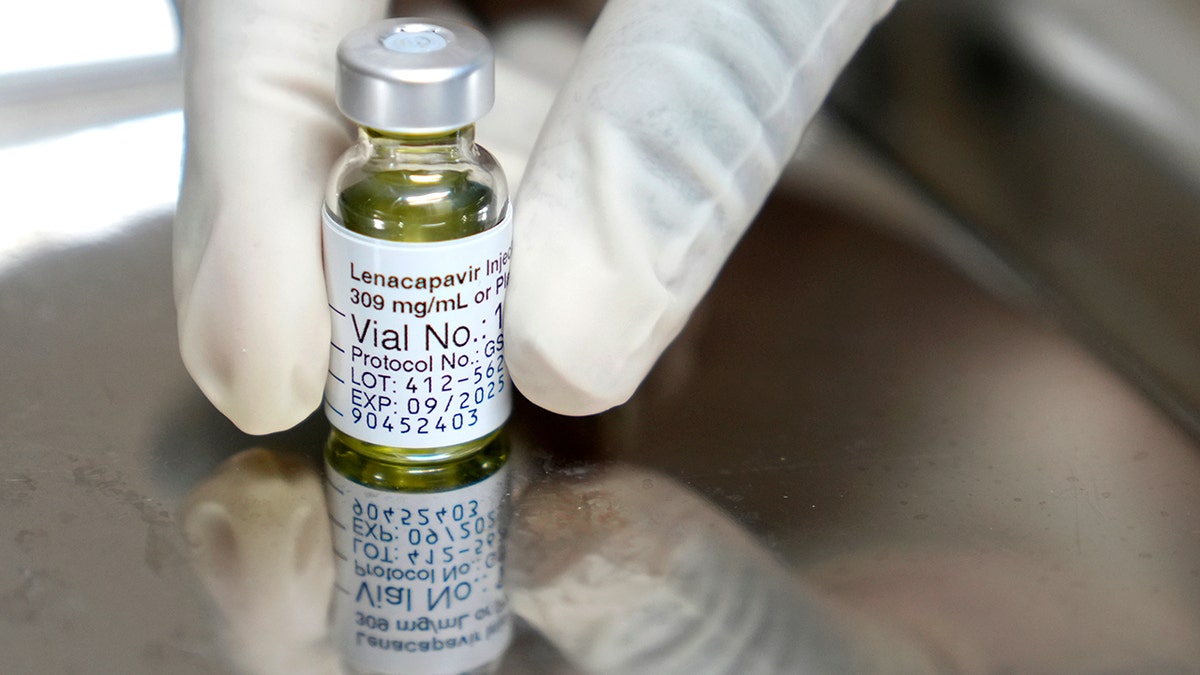
This drug is administered as injectable under the skin that the body slowly absorbs. Individuals should undergo a negative HIV-1 test before starting treatment. (AP Photo / Nardus Angelled)
Journal Science was named the Renacapaville, 2024’s “Breakthrough of the Year.”
Click here to sign up for our health newsletter
Lenacapavir uses a multi-stage approach that distinguishes it from other approved ones Antiviral drugs.

The company’s injectable HIV-1 Capsid inhibitor (Lenacapavir), sold under the name Yeztugo, reduces the risk of sexually acquired HIV in adults and adolescents. (istock)
“Most antiviral agents work in one stage Virus ReplicationRenacapavil is designed to inhibit HIV at multiple stages of the life cycle,” said a press release from Gilead.
Visit us for more health articles www.foxnews.com/health
“Yeztugo is one of the most important scientific breakthroughs of our time and offers a very realistic opportunity to help end the HIV epidemic,” O’day said in a press release.
The most commonly reported side effects during clinical trials included injection site reaction, headaches and nausea, according to the company.






